Question :
What are proteins? Write a detailed account of proteins and their importance.
Answer:
PROTEINS:
Proteins are the most abundant organic compounds to be found in cells comprise over 50% of their total dry weight. They are present in all types of and in all parts of the cell. Functions of Proteins
1. Structural Component: They build many structures of the cell e.g. plasma membrane.
2. Enzymes: All enzymes are proteins and in this way they control the whole metabolism of the cell.
3. Keratin: Skin, nails, hair, feathers, horn etc. contain protein called keratin.
4. Viral Protein Coat: Viruses are sub cellular in structure and possess outer coat of protein.
5. Storage Protein: Casein is the milk protein and albumin is the egg white protein.
6. Collagen: Collagen present in bones, cartilage, etc. is the most abundant protein in higher vertebrates.
7. Hormones: As hormones, proteins regulate metabolic processes.
8. Transport: Some proteins (e.g. heamoglobin) work as carriers and transport specific substances such as oxygen, lipids, metal ions, etc.
9 Antibodies: Some proteins, called antibodies, defend the body against pathogens.
10. Blood Clotting: Blood clotting proteins prevent the loss of blood from the body after an injury.
11. Muscle Protein: Muscles are also composed of proteins.
12. Contractile protein & Muscle Contraction: Proteins such as actin and myosin play key role in muscle contraction.
13. Contractile protein & spindle fibers: Proteins cause movement of chromosomes during anaphase of cell division.
CHEMICAL COMPOSITION OF PROTEINS:
Proteins are polymers of amino acids, the compounds containing carbon, nitrogen, oxygen and hydrogen. The number of amino acids varies from a few to 3000 or even more in different proteins.
AMINO ACIDS: About 170 types of amino acids have been found to occur in cells and tissues. Of these, about 25 are constituents of proteins. Most of the proteins are however, made of 20 types of amino acids.
Structure of an amino acid: All the amino acids have an amino group (-NH,) and a carboxyl group (-COOH) attached to the same carbon atom, also known as alpha carbon.
They have the general formula as:
1. A hydrogen atom
2. An amino (NH,) group
3. A carboxyl group(COOH)
4. "Something else", this is the "R" group.
R may be a H as in glycine, or CH3 as in alanine, or any other group. So amino acids mainly differ in the R group.
Polypeptides: Amino acids are linked together to form polypeptides of proteins. The amino group of one amino acid may react with the carboxyl group of another releasing a molecule of water.
For example: glycine and alanine may combine to form a dipeptide.
Linkage: The linkage between the hydroxyl group of carboxyl group of one amino acid and the hydrogen of amino group of another amino acid release H20 and C N link to forma bond called peptide bond. The resultant compound glycylalanine has two amino acid subunits and is a dipeptide. A dipeptide has an amino group at one end and a carboxyl group at the other end of the molecule. So both reactive parts are again available for further peptide bonds to produce tripeptides, tetrapeptides, pentapeptides and polypeptides, leading to polypeptide chains.
STRUCTURE OF PROTEINS: Each protein has specific properties, which are determined by the number and the specific sequence of amino acids in a molecule, and upon the shape, which the molecule assumes as the chain folds into its final, compact form.
Levels of Organization: There are four levels of organization, which are described below.
PRIMARY STRUCTURE: The primary structure composes the number and sequence of amino acids in a protein molecule. E. Sanger was the first scientist who determined the sequence of amino acids in a protein molecule.
Structure of Insulin: After ten years of careful work, he concluded in 1954, that insulin is composed of 51 amino acids in two chains. One of the chains had 21 amino acids and the other had 30 amino acids and di-sulphide bridges held them together.
Structure of Haemoglobin: Haemoglobin is composed of four chains, two alpha and two beta chains. Each alpha chain contains 141 amino acids, while each beta chain contains 146 amino acids.
Size & Shape of proteins: The type of amino acids and the number of amino acids comprising that particular protein molecule determines the size of a protein molecule. Polypeptide chains in keratin (fibrous) and in hemoglobin (globular) are held together to form respective functional proteins. Now we know that there are over 10,000 proteins in the human body, which are composed of unique and specific arrangements of 20 types of amino acids. The sequence is determined by the order of nucleotides in the DNA.
Arrangement of amino acids: The arrangement of amino acids in a protein molecule is highly specific for its proper functioning. If any amino acid is not in its normal place, the protein fails to carry on its normal function. The best example is the sickle cell hemoglobin of human beings. In this case only one of the 574 amino acids do not occupy the normal place in the proteins (in fact this particular amino acid is replaced by some other amino acid), and the haemoglobin fails to carry any or sufficient oxygen, hence leading to death of the patient.
SECONDARY STRUCTURE:
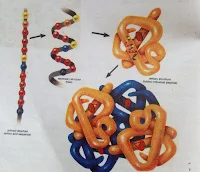
Arrangement of polypeptide: The polypeptide chains in a protein molecule usually do not lie flat. They usually coil into a helix, or into some other regular configuration.
Helix: One of the common secondary structures is the -helix. It involves a spiral formation of the basic polypeptide chain. The -helix is a very uniform geometric structure with 3.6 amino acids in each turn of the helix. The helical structure is kept by the formation of hydrogen bonds among amino acid molecules in successive turns of the spiral.
TERTIARY STRUCTURE: Usually a polypeptide chain bends and folds upon itself forming a globular shape. This is the proteins' tertiary conformation. It is maintained by three types of bonds, namely ionic, hydrogen, and disulfide (-S-S-).
For example: in aqueous environment the most stable tertiary conformation is that in which hydrophobic amino acids are buried inside while the hydrophilic amino acids are on the surface of the molecule.
QUATERNARY STRUCTURE: In many highly complex proteins, polypeptide tertiary chains are aggregated and held together by hydrophobic interactions, hydrogen and ionic bonds. This specific arrangement is the quaternary structure. Haemoglobin, the oxygen carrying protein of red blood cells, exhibits such a structure.
CLASSIFICATION OF PROTEINS: According to their structure, proteins are classified as follows:
1. Fibrous proteins: They consist of molecules having one or more polypeptide chains in the form of fibrils. Secondary structure is most important in them. They are insoluble in aqueous media. They are non-crystalline and are elastic in nature. They perform structural roles in cells and organisms.
Examples: are silk fiber (from silk worm, and spiders' web) myosin (in muscle cells), fibrin (of blood clot), and keratin (of nails and hair).
2. Globular proteins: These are spherical or ellipsoidal due to multiple folding of polypeptide chains. Tertiary structure is most important in them. They are soluble in aqueous media such as salt solution, solution of acids or bases, or aqueous alcohol. They can be crystallized. They disorganize with changes in the physical and physiological environment.
Examples: are enzymes, antibodies, hormones and haemoglobin.
Pre-medical ist year biology notes





0 Comments